Scroll to:
Peculiarities of management of children with intestinal stomas of different localization in the neonatal period
https://doi.org/10.37489/2949-1924-0101
EDN: MBYUNI
Abstract
Intestinal stoma formation in neonates with severe gastrointestinal diseases or congenital malformations remains a critical and challenging issue in neonatology. Postoperatively, preventing associated complications-such as high enteric stoma losses, electrolyte imbalances, malabsorption syndrome, cholestasis, and small intestinal bacterial overgrowth — is equally urgent. Effective neonatal resuscitation with intensive stabilization, comprehensive monitoring, homeostasis correction, and infection control is essential. Early initiation of enteral nutrition (starting with trophic feeds and gradually advancing based on the preserved bowel’s capacity) alongside parenteral support is crucial post-surgery. Breast milk provision and maternal involvement in stoma care are vital components. The ultimate goal of management is to prepare the infant for definitive reconstructive surgery.
For citations:
Kupriyanova E.A., Mirzoeva Ya.S., Siluyanova N.Yu., Stroeva L.E. Peculiarities of management of children with intestinal stomas of different localization in the neonatal period. Patient-Oriented Medicine and Pharmacy. 2025;3(3):40-49. (In Russ.) https://doi.org/10.37489/2949-1924-0101. EDN: MBYUNI
Relevance
Neonatal emergency pathology requiring life-saving intestinal stoma formation is a pressing issue in neonatology and neonatal surgery. A particular focus within this field is the management strategy for newborns with small intestinal stomas and the potential risk of short bowel syndrome. The term "stoma" (from Greek ostomy) refers to a surgically created opening connecting the lumen of an internal organ (in this case, the intestine) to the surface of the body. Indications for intestinal stomas in newborns include severe gastrointestinal (GI) tract diseases and/or congenital malformations. The management strategy for children with intestinal stomas involves choosing the optimal surgical technique, ensuring early restoration of GI tract content passage, preventing complications, and providing meticulous postoperative care, including stoma care. It is crucial to ensure adequate nutrition for optimal infant growth; minimize losses of fluid, nutrients, and electrolytes; and create the most favorable conditions possible for intestinal adaptation. This is especially critical for children at risk of developing intestinal and/or hepatocellular failure. Age-appropriate development of newborns, along with the prevention and correction of postoperative complications, is an essential part of preparing for the reconstructive phase of treatment and ensuring future quality of life.
Objective
To summarize the experience in managing newborns with intestinal stomas at different locations and to define directions for early systemic rehabilitation in the postoperative period. This aims to optimize the adaptation of the GI tract and the body as a whole to the altered living conditions, which will form the basis for better infant growth and development.
Materials and Methods
A retrospective analysis was conducted of 16 medical records of newborns treated in the ICU of the Yaroslavl Regional Children's Clinical Hospital between 2019 and 2024. Term newborns constituted 56% (n=9), while preterm infants constituted 44% (n=7: with gestational ages of 28–31 weeks – three, 34–36 weeks – four). Girls and boys were represented almost equally, at 56% and 44% respectively (n=9 and 7). Associated pathology in every third child was birth asphyxia: moderate in 31% (n=5), severe in 6% (n=1). Four patients were diagnosed with congenital heart defects (VSD, ASD, anomalous pulmonary venous drainage). Another four had hydronephrosis.
Results and Discussion
An intestinal stoma was formed in all patients for life-saving reasons: in 75% (n=12) during the early neonatal period, and in 25% (n=4) during the late neonatal period. Indications for surgical intervention and stoma creation were: congenital intestinal malformation – ileal stenosis with intestinal obstruction – 6% (n=1), anal atresia – 45% (n=7), Ladd's syndrome 6% (n=1), Hirschsprung's disease 6% (n=1), meconium ileus 6% (n=1), congenital peritonitis 6% (n=1), enterocolitis – 25% (n=4). Out of 12 cases of congenital intestinal pathology, six (50%) were detected prenatally. Most patients experienced non-surgical complications in the postoperative period: cholestasis (31%, n=5), malabsorption syndrome (19%, n=3), small intestinal bacterial overgrowth with reduced intestinal reparative capacity (25%, n=4). There were two surgical complications (12%): one (6%) newborn developed intestinal bleeding in the early postoperative period, and one (6%) was found to have insufficiency of one of the intestinal anastomoses during postoperative care. All infants (100%) experienced difficulties with breastfeeding, primarily related to the stress experienced by mothers due to the child's illness, as well as the specifics of feeding the child, the need for milk expression, etc.
Table 1. Location of Stoma for Various Pathologies in Newborns
| Disease / Congenital Malformation | Most Common Stoma Location |
|---|---|
| Intestinal Atresia | Duodenum, Ileum or Jejunum |
| Anal Atresia | Large Intestine |
| Ladd's Syndrome | Ileum or Jejunum |
| Meconium Ileus | Ileum |
| Hirschsprung's Disease | Sigmoid Colon |
| Necrotizing Enterocolitis | Ileum or Jejunum |
According to the literature, in patients with stomas, the effectiveness of care, prognosis, and negative short- and long-term consequences are closely related to the length and function of the resected or excluded intestinal segment [1, 2]. Figure 1 schematically shows the locations of various intestinal stomas. Depending on the level of stoma formation, the following types are distinguished: enterostomy (a stoma created from the small intestine), jejunostomy (from a loop of the jejunum in the upper part of the small intestine), ileostomy (from a loop of the ileum), colostomy (connects the large intestine to the anterior abdominal wall) [3]. In our study, 50% (n=8) of patients had a small intestinal stoma, and 50% (n=8) had a colostomy. Specifically, jejunostomy was performed in two infants, ileostomy in one, and sigmoidostomy in three.
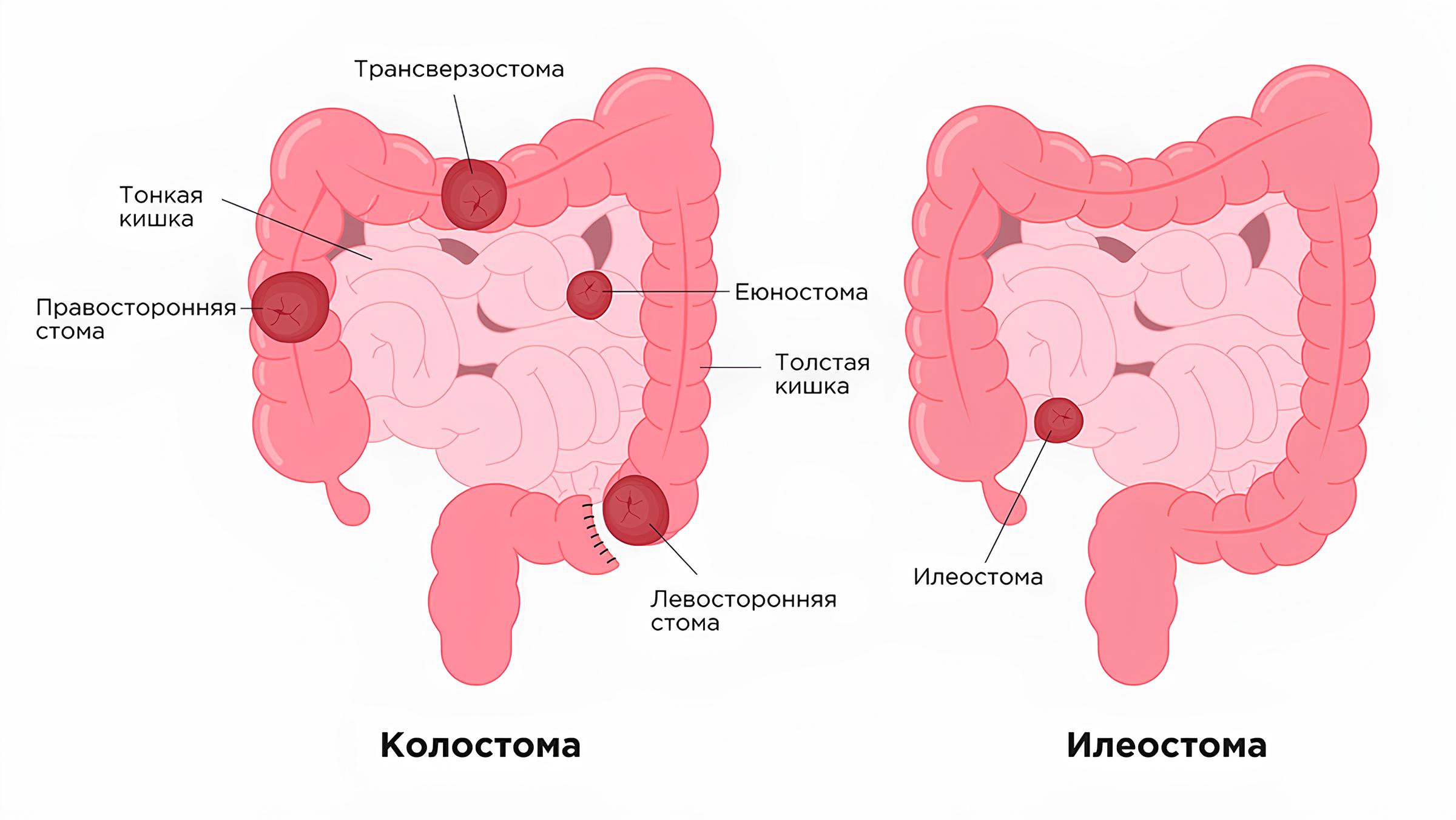
Fig. 1: Schematic diagram of the intestine and stoma locations
The ileocecal region of the large intestine, shown in Fig. 2, is multifunctional. It, in particular, regulates intestinal motor activity. The ileocecal valve – the Bauhin's valve – is equipped with circular muscles, hence a number of authors consider it a sphincter [4]. Removal of the ileocecal valve leads to retrograde migration (translocation) of the bacterial flora from the large intestine and its excessive proliferation [5].
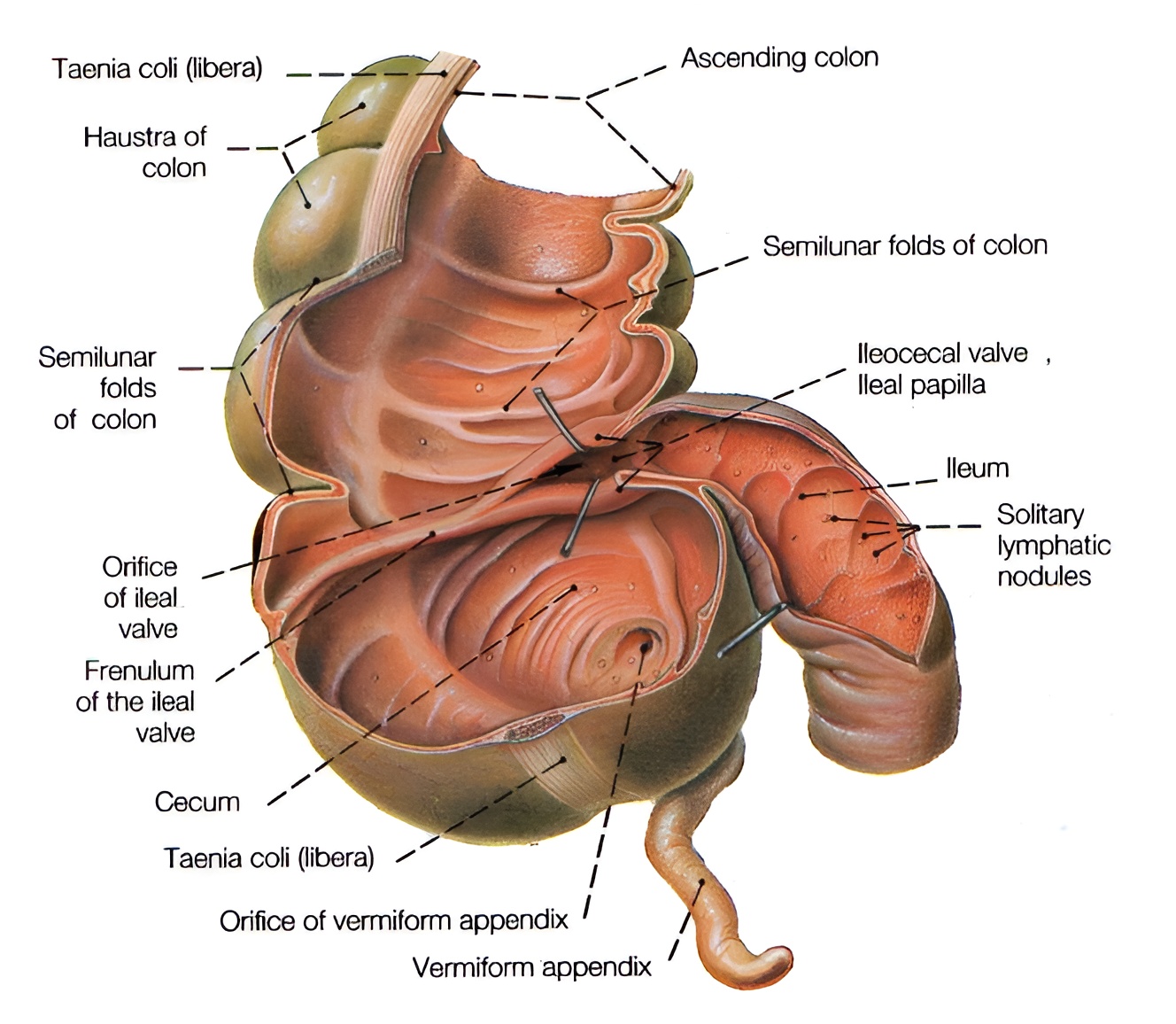
Fig. 2: Cecum with the ileocecal orifice, featuring a valve
Resection of the large intestine causes accelerated transit of contents through the intestine, loss of fluid and electrolytes, and dehydration.
One of the complex problems is the management strategy for newborns with small intestinal stomas. After resection of various parts of the small intestine (duodenum, jejunum and/or ileum), the absorption of essential nutrients (proteins, fats, carbohydrates), as well as fluid, electrolytes, and bile acids, is impaired [6]. With a jejunostomy (from a loop of the jejunum in the upper small intestine), particularly significant loss of intestinal chyme is observed, often exceeding the nutritional load, which progressively disrupts the natural digestive process and leads to decompensation of the general condition [7].
Nutritional support for patients after surgical treatment, as recommended [8], included a combination of parenteral and enteral nutrition. Three stages are mandatory: the first – total parenteral nutrition – was conducted when enteral loading was impossible due to impaired GI motility or the need for bowel "rest," for example, in the early postoperative period. The second stage – partial parenteral nutrition with gradual reintroduction of enteral nutrition. The third stage – transition, as much as possible, to enteral nutrition, which primarily depends on the length of the remaining intestine [9].
The calculation of nutrient requirements and parenteral nutrition in our sample were performed in accordance with the draft clinical protocol "Parenteral Nutrition of Newborns" [10]. Children were prescribed an additional fluid volume of 10–12 ml/kg/day. The amount of protein was calculated, as recommended, based on weight and gestational age (2.5–3.0 g/kg/day), with a significant increase (up to 4–4.5 g/kg/day) in severely ill patients with concomitant cardiorespiratory problems, critical conditions, and systemic inflammatory response.
Initiating enteral nutrition is a complex and crucial moment in the postoperative care of newborns with stomas. For better intestinal adaptation, it is necessary to strive for its early initiation with trophic feeds, conduct it continuously, and monitor the effectiveness of feeding and the maintenance of tolerance to the nutritional load. In our patients, the restoration of intestinal passage and the appearance of stool usually occurred on the 3rd–4th day after surgery. By this time, it was possible to stabilize hemodynamics, resolve respiratory disorders, and alleviate postoperative intestinal paresis. Parenteral nutrition, as recommended [10, 11, 12], was reduced depending on the amount of nutrients and calories the child could consume and absorb enterally each day, which primarily depended on the length of the small intestine deemed viable and preserved during surgery. It is believed that if the ileocecal valve is functional and the length of the small intestine is at least 25 cm, or if 40–50 cm of small intestine remains in the absence of the Bauhin's valve, then the infant has a chance of weaning off total parenteral nutrition [13]. Even in the latter case, early initiation of trophic feeding allows for discontinuation of total parenteral nutrition in half of the patients [14]. Starting with trophic feeding at 1–5 ml/kg/day, the amount of offered food was increased by 10–20 ml/kg/day according to tolerance, up to the full physiological volume. As recommended, the progression of intestinal contents and chyme losses were monitored; these losses should not exceed 30–40 ml/kg/day (occurring primarily through the stoma) [15]. The speed of weaning from parenteral nutrition mainly depended on the losses from the intestinal stoma, the length of the remaining small intestine, and the percentage of daily energy and nutrient requirements it could provide. Recommended monitoring was mandatory [15]. The patient's diuresis, urine tests, and serum indicators were monitored: electrolytes, total protein, urea, total bilirubin and its fractions, cholestasis enzymes (GGT, ALP), hepatocyte cytolysis markers (ALT, AST), coagulogram, complete blood count, CRP.
Unconditional preference in enteral nutrition should be given to breast milk [16]. It contains not only a complete composition of nutrients but also immune defense factors for the infant, essential long-chain fatty acids (including anti-inflammatory ones), medium-chain fatty acids with high energy value and easy digestibility, and extremely important growth factors that stimulate intestinal adaptation and repair, among many other valuable components [17, 18, 19]. There is evidence that the use of breast milk reduces the duration of parenteral nutrition due to accelerated intestinal adaptation to the enteral load [20, 9].
In our study, 56% of infants (n=9) received breast milk in the early postoperative period. For the remaining 44% (n=7), as recommended [9], therapeutic formulas with extensively hydrolyzed cow's milk protein, lactose-free, and enriched with medium-chain fatty acids were used. The negative impact of an enteral feeding pause, especially a prolonged one, is known. Although there is still no conclusive research on the optimal and maximum possible duration of such a pause, most authors believe it should not exceed 5–7 days [21]. Prolonged absence of enteral nutrition is accompanied by reduced production of enteral hormones (cholecystokinin, motilin, gastrin, etc.), disruption of the enterohepatic circulation of bile acids, which can lead to cholestasis and Intestinal Failure Associated Liver Disease (IFALD) [22]. Figure 3 illustrates the synthesis and enterohepatic circulation of bile acids, which is impaired in cholestasis.
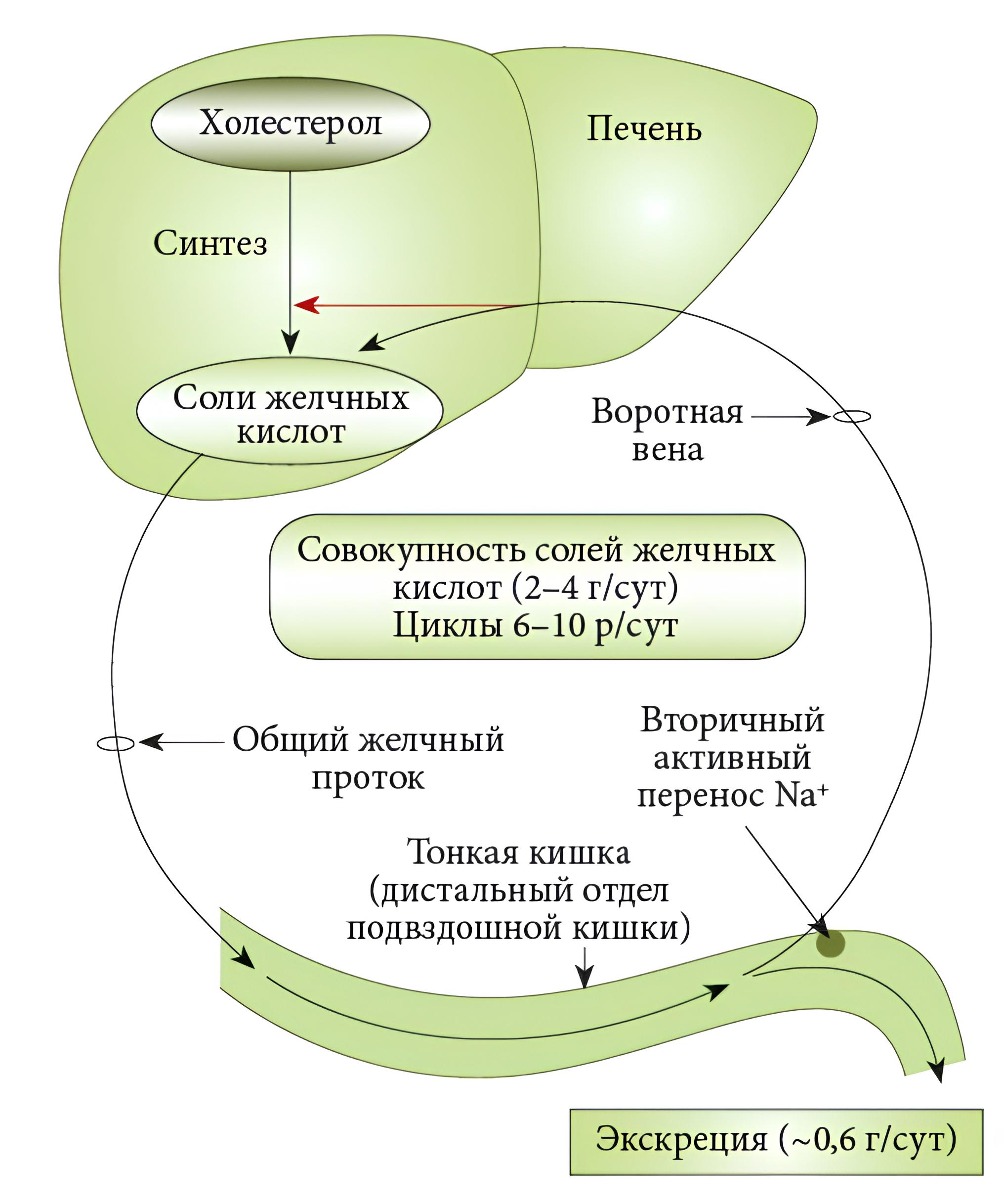
Fig. 3: Diagram of bile acid synthesis and enterohepatic circulation, impaired in cholestasis
Cholestasis syndrome can be diagnosed based on an increase in direct bilirubin to more than 20% of the total level or above 17 µmol/L, combined with increased activity of alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), cholesterol and bile acids, as well as a decreased prothrombin index. We confirm that during prolonged parenteral nutrition, limiting the use of intravenous fat emulsions based on soybean lipids reduces cholestasis [23]. When signs of cholestasis were detected, and until its resolution, as recommended [11], our patients received oral ursodeoxycholic acid at a dose of 20 mg/kg/day, divided into two doses.
In 25% (n=4) of newborns with stomas, a clinical syndrome of bacterial translocation and overgrowth was observed, manifested by abdominal distension, tension of the abdominal wall, impaired intestinal passage (absence of peristalsis and discharge from the stoma), bilious vomiting; inflammatory markers in blood tests were elevated. In such cases, a cascade of pro-inflammatory reactions can be triggered, competition of microorganisms for metabolites occurs, consuming enteral nutrients and vitamins, leading to tissue hypoxia, deconjugation of bile acids, accumulation of toxic metabolites, and worsening repair of the operated intestinal segment [5]. The strategy for preventing bacterial overgrowth includes the use of antibiotics and reducing carbohydrate intake in patients on partial enteral nutrition. To correct this complication in the postoperative period, all our patients received antibiotic therapy; in the mentioned 25% (n=4), the course of intestinal decontamination was repeated.
Stomas in newborns are almost always temporary. Although the timing and methods of their closure and anastomosis formation are highly debatable, after some time (3–6 months or significantly earlier) the stoma will be closed, and defecation will be normal [7, 24]. Despite the relatively short period of contact between intestinal contents and the abdominal skin, stoma care is not a simple matter. Even in term newborns, the stratum corneum is very thin (about 30% thinner than in adults), so erosions, skin maceration, peristomal dermatitis, and bacterial inflammation are highly likely to occur around the stoma. The skin can also be damaged due to allergic reactions to components of stoma bags. According to most authors, the specifics of stoma care and complications depend on the stoma formation method and, of course, on the section of the stoma-intestine [7, 24]. In this regard, it is important to explain to parents the anatomical features of the stoma, draw attention to predictors of complications requiring medical attention, and teach proper home care [25]. It has been shown [26] that mothers provided better care for children at home than in a hospital setting. It is necessary to individually and timely select means and materials for caring for the skin around the stoma. Special wipes and ointments, gels, protective film-forming agents are used, for example, the protective film from Coloplast, individually packaged wipes from Conveen, etc. When using such wipes, a protective layer, an elastic film, forms after a minute or two, which can later be removed with water. Special cleansing and degreasing agents for care are also used, for example, from Coloplast or ConvaTec. Barrier pastes, mainly based on zinc oxide, are also used. Manufacturing companies ("Coloplast" and "ConvaTec") produce stoma bags that can be used from the neonatal period [25]. When using a stoma bag, regular air baths are beneficial for preventing and treating peristomal complications. Products that can disrupt the skin barrier function and change its pH are not recommended, such as soap, baby wipes, as well as products containing alcohol, oils, etc.
To demonstrate the management tactics for a newborn with an ileostomy, we present the clinical case of patient K.
The child was from the fifth pregnancy, preceded by two births and 2 medical abortions. In the first trimester, the woman had an acute respiratory viral infection with high fever and antibiotic therapy. She was under dispensary observation from 32 weeks; gestational diabetes and polyhydramnios were detected. Delivery at 37 weeks with premature rupture of clear amniotic fluid. The interval without waters was 6 hours. The first stage of labor lasted 2 hours 45 minutes, the second – 10 minutes. Apgar score 9/9. Birth weight 3115 g, length 50 cm.
From birth, the condition was assessed as satisfactory. Deterioration occurred towards the end of the first day of life: hyperexcitability, irritated monotonous crying, early jaundice, grayish skin tone, acrocyanosis. The abdomen was tense, the liver was enlarged, and scanty stool was obtained only after an enema. At 25 hours of life, the child was admitted to the neonatal resuscitation unit in severe condition: mechanical ventilation was initiated, he received antibiotic therapy, and total parenteral nutrition.
At 37 hours of life, with a diagnosis of intestinal obstruction, perforation of a hollow organ, he was transferred for surgical care to the neonatal intensive care unit of the regional children's clinical hospital in severe condition on mechanical ventilation. Abdominal X-rays revealed the "sickle" sign characteristic of pneumoperitoneum (Fig. 4).
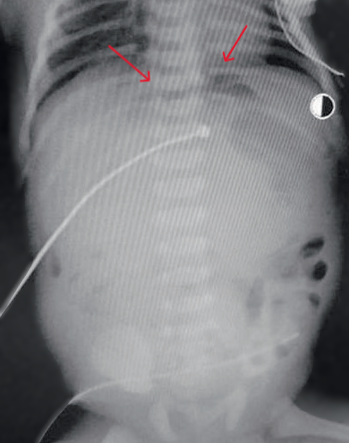
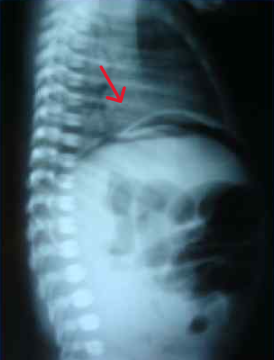
Fig. 4: Abdominal X-ray showing the characteristic "sickle" sign of pneumoperitoneum
An emergency laparotomy was performed. During exploration of the abdominal cavity, a significant amount of turbid exudate with pus was found, loops of the small intestine were distended, hyperemic, purplish with fibrin deposits, edematous, with loose adhesions. The loops contained a significant amount of meconium; several areas of the terminal ileum had thickenings, one of them narrowed (a closed antenatal perforation), no visible perforation was found. The abdominal cavity was drained. An ileostomy was created.
Postoperative diagnosis of the underlying disease: Infection specific to the perinatal period: intrauterine perforative enterocolitis, purulent peritonitis. Operation: Laparotomy, exploration and drainage of the abdominal cavity, ileostomy.
In the postoperative period, the child's condition remained severe. Clinical and paraclinical signs of bacterial infection were pronounced, intestinal paresis, hemodynamic instability, and hemorrhagic syndrome with pulmonary hemorrhage were noted, likely caused by liver damage associated with infection and the absence of enteral nutrition. He received mechanical ventilation, parenteral nutrition, massive antibiotic therapy, cardiotonics, transfusions of fresh frozen plasma, and red blood cell suspension. Sustained improvement occurred by the 8th day after surgery: respiratory support was discontinued, intestinal peristalsis appeared, and watery yellow-green stool began to pass through the stoma. The next day, enteral feeding was started in trophic volumes (1–5 ml/kg/day) with gradual, slow expansion.
At 30 days of age, the child, who had been an ileostomy carrier since the second day of life, having suffered intrauterine perforative enterocolitis and purulent peritonitis, and having nutritional insufficiency against the background of short bowel syndrome, underwent a relaparotomy: revision of the abdominal cavity, resection of the ileocecal angle (granulomatous colitis with perforation found in the operative biopsy material), formation of an ileocolonic anastomosis and an ileoileal anastomosis.
The clinical case shows that in a newborn, the exclusion of a large part of the ileum affected by a congenital infection and the creation of an ileostomy within the first two days of life was a life-saving operation. Despite the severe course of the underlying disease (intrauterine perforative enterocolitis and purulent peritonitis), the need to combat infection, typical non-surgical complications, intensive postoperative therapy in the neonatal ICU, parenteral and relatively early enteral nutrition, including breast milk, and adequate care allowed for the transition to the reconstructive stage of treatment within just one month.
Conclusion
Intestinal stoma formation, especially of the small intestine, in newborns with severe diseases and malformations of the gastrointestinal tract is a relevant and complex problem in neonatology. For the surgeon, the choice of method and level for stoma formation, as well as the timing of its closure, is not straightforward. After surgery, an equally acute problem is the prevention of associated complications. More often these are non-surgical complications (significant enteral losses through the stoma, electrolyte disturbances, malabsorption syndrome, cholestasis, small intestinal bacterial overgrowth), which reduce the reparative capabilities of the intestine and contribute to the development of chronic intestinal and liver failure.
Adequate resuscitation care for the newborn with intensive stabilization, comprehensive monitoring, correction of homeostasis, and infection control is very important. It is necessary, along with parenteral nutrition, to start trophic enteral nutrition as early as possible and expand it according to the tolerance of the preserved intestine. It is especially important to have breast milk and maternal involvement in the care of the infant and the stoma.
The prevention and timely correction of complications associated with stoma formation are priority tasks in the care and preparation of the child for the final reconstructive stage of treatment.
References
1. Ivanov S.D., Slizovskij G.V., Balaganskiy D.A, et al. Outcomes of surgical treatment of neonates with intestinal stomas in a regional perinatal center. Detskaya khirurgiya (Russian Journal of Pediatric Surgery) 2021; 25(3): 158-164. (In Russ.) doi: 10.18821/1560-9510-2021-25-3-158-164
2. Minaev S.V., Bykov N.I., Isaeva A.V. et al. The complications of intestinal stoma in children. Pirogov Russian Journal of Surgery. 2017;(1):54-57. (In Russ.) doi: 10.17116/hirurgia2017154-57
3. Naumova N.A., Leontyeva E.A. Nuances of Managing Patients with Inflammatory Bowel Diseases. First Medical TV Channel. Lecture. 2023.
4. Valker F.I. The Development of Human Organs After Birth. Leningrad: Medgiz, 1951. 190 p.
5. Korpela K, Mutanen A, Salonen A, Savilahti E, de Vos WM, Pakarinen MP. Intestinal Microbiota Signatures Associated With Histological Liver Steatosis in Pediatric-Onset Intestinal Failure. JPEN J Parenter Enteral Nutr. 2017 Feb;41(2):238-248. doi: 10.1177/0148607115584388.
6. Averyanova Yu.V., Batyrshin E.M., Demko A.E. et al. Clinical recommendations of the Northwest Society for Enteral and Parenteral Nutrition, Interregional Association for Emergency Surgery, Russian Gastroenterological Association, Union of Rehabilitation Therapists of Russia and Russian Transplantation Society on diagnosis and treatment of short bowel syndrome-associated intestinal failure in adults. Russian Journal of Gastroenterology, Hepatology, Coloproctology. 2022;32(1):60–103. doi: 10.22416/1382-4376-2022-32-1-60-103.
7. Ivanov S.D., Slizovskij G.V., Shikunova J.V. Enterostomy in neonates : relevant review of surgical treatment. Ros Vestn Perinatol i Pediatr 2022; 67:(1): 21–27 (in Russ.). doi: 10.21508/1027-4065-2022-67-1-21-27
8. Yerpulyova, Yu.V., Chubarova, A.I., Chugunova, O.L. (Eds.). (2016). Parenteral and enteral nutrition in children : Practical guidelines; GEOTAR-Media 2016.
9. Chubarova A.I. Therapeutic enteral and parenteral nutrition of newborns. National Guidelines. Moscow: GEOTAR-Media; 2014;653-692.
10. Grosheva EV, Degtyarev DN, Ionov OV, et al. Draft clinical protocol "Parenteral nutrition of newborns". Neonatologiya: novosti, mneniya, obuchenie 2013;(2):89-97.
11. New D. Gastroenterology and Nutrition: Neonatology Challenges and Controversies [Russian translation edited by Yu.G. Mukhina]. Moscow: Logosphere; 2014.
12. Belza C, Fitzgerald K, de Silva N, Avitzur Y, Steinberg K, Courtney-Martin G, Wales PW. Predicting Intestinal Adaptation in Pediatric Intestinal Failure: A Retrospective Cohort Study. Ann Surg. 2019 May;269(5):988-993. doi: 10.1097/SLA.0000000000002602.
13. Isakov YuF, Volodin NN, Geraskin AV. Neonatal Surgery. Moscow: Dinastiya; 2011. 680 p.
14. Averyanova Yu.V., Vessel L., Erpulyova Yu.V. et al. Federal clinical recommendations «treatment of children with the short bowel syndrome». Russian Journal of Pediatric Surgery, Anesthesia and Intensive Care. 2014;4:92-108.
15. Erpuleva Yu.V., Chubarova A.I. Modern Management of Children with Short Bowel Syndrome and Other Forms of Chronic Intestinal Failure. A Guide for Physicians. Moscow: GEOTAR-Media, 2016. 150 p.
16. Chubarova A.I. Algorithm for Selecting Enteral Nutrition in Newborns with Surgical Diseases of the Intestine. In: Proceedings of the 5<sup>th</sup> Congress of the Scientific Society of Gastroenterologists of Russia and the 32<sup>nd</sup> Session of the Central Research Institute of Gastroenterology. Moscow, 2005. P. 507.
17. Skvortsova V.A., Borovik T.E., Netrebenko O.K. et al. The role of breast milk in nutrition and care of preterm infants. Pediatrics. Journal named after G.N. Speransky. 2015;5
18. Patel AL, Johnson TJ, Engstrom JL, et al. Impact of early human milk on sepsis and health-care costs in very low birth weight infants. J Perinatol. 2013 Jul;33(7):514-9. doi: 10.1038/jp.2013.2.
19. Ben XM, Li J, Feng ZT, Shi SY, Lu YD, Chen R, Zhou XY. Low level of galacto-oligosaccharide in infant formula stimulates growth of intestinal Bifidobacteria and Lactobacilli. World J Gastroenterol. 2008 Nov 14;14(42):6564-8. doi: 10.3748/wjg.14.6564.
20. Ou J, Courtney CM, Steinberger AE, Tecos ME, Warner BW. Nutrition in Necrotizing Enterocolitis and Following Intestinal Resection. Nutrients. 2020 Feb 18;12(2):520. doi: 10.3390/nu12020520.
21. Grosheva E.V., Zubkov V.V. Management of enteral nutrition of premature newborns who have undergone conservative necrotizing enterocolitis as the basis for favorable short and long-term outcomes. Neonatologiya: novosti, mneniya, obuchenie. 2021;9(2):33–9. doi: 10.33029/2308-2402-2021-9-2-33-39 (in Russ.)
22. Bishay M, Pichler J, Horn V, Macdonald S, Ellmer M, Eaton S, Hill S, Pierro A. Intestinal failure-associated liver disease in surgical infants requiring long-term parenteral nutrition. J Pediatr Surg. 2012 Feb;47(2):359-62. doi: 10.1016/j.jpedsurg.2011.11.032.
23. Erpuleva Yu.V., Chubarova A.I., Vainshtein N.P. et al. Complications of long-term parenteral nutrition in newborns and infants. Russian Journal of Pediatric Surgery, Anesthesia and Intensive Care. 2017;7(1):59-69.
24. Ionov A.L., Shcherbakova O.V., Sulavko Ya.P. et al. Peristomal complications and principles of intestinal stoma care in children. Russian Journal of Pediatric Surgery, Anesthesia and Intensive Care. 2011;4:3-11.
25. Logunova Yu. What to Do Next: A Brief Guide to Stoma Care for Parents of Special Needs Children and Junior to Intermediate Medical Staff. Moscow: R. Valent; 2014. 124 p.
26. Melville CA, Bisset WM, Long S, Milla PJ. Counting the cost: hospital versus home central venous catheter survival. J Hosp Infect. 1997 Mar;35(3):197-205. doi: 10.1016/s0195-6701(97)90207-3.
About the Authors
E. A. KupriyanovaРоссия
Ekaterina A. Kupriyanova, pediatrician, resident neonatologist
IPO; Department of Pediatrics
Yaroslavl
Competing Interests:
Authors declare no conflict of interest requiring disclosure in this article
Ya. S. Mirzoeva
Россия
Yana S. Mirzoeva, therapist, resident neonatologist
IPO; Department of Pediatrics
Yaroslavl
Competing Interests:
Authors declare no conflict of interest requiring disclosure in this article
N. Yu. Siluyanova
Россия
Natalia Yu. Siluyanova, assistant, Head of the Unit, Chief External Neonatology Specialist, Ministry of Health of the Yaroslavl Region
IPO; Department of Pediatrics; Department of Phthisiology; Neonatal Intensive Care Unit
Yaroslavl
Competing Interests:
Authors declare no conflict of interest requiring disclosure in this article
L. E. Stroeva
Россия
Larisa E. Stroeva, Cand. Sci. (Med.), Associate Professor
IPO; Department of Pediatrics
Yaroslavl
Competing Interests:
Authors declare no conflict of interest requiring disclosure in this article
Review
For citations:
Kupriyanova E.A., Mirzoeva Ya.S., Siluyanova N.Yu., Stroeva L.E. Peculiarities of management of children with intestinal stomas of different localization in the neonatal period. Patient-Oriented Medicine and Pharmacy. 2025;3(3):40-49. (In Russ.) https://doi.org/10.37489/2949-1924-0101. EDN: MBYUNI
JATS XML


































.png)
