Scroll to:
Digitalization of pharmaceutical care for children with rare diseases
https://doi.org/10.37489/2949-1924-0109
EDN: TTNEGJ
Abstract
Background. Pharmaceutical care for children with rare diseases is an integral part of the state-sponsored drug benefit system and is facilitated through the Circle of Kindness Foundation. The digitalization and standardization of business processes for the circulation of medicines will help eliminate practical challenges and mitigate risks in managing treatment for these children at the regional level.
Methods. The study employed a set of scientific methods, including system, logical, and structural analysis. The objects of the study were regulatory legal acts, the International Classification of Diseases (ICD), the State Register of Medicines, the Unified Register of Registered Medicines of the Eurasian Economic Union, inventory management systems, and analytical data
from the official websites of government agencies regulating rare diseases and the "Circle of Kindness" Foundation for Support of Children with Severe Life-Threatening and Chronic Diseases.
Results. Regional-specific aspects of digitalizing drug provision for children with rare diseases were identified.
Conclusion. Enhancing the regulatory framework for digital transformation and standardizing the list of unregistered medicines based on digital information from barcodes or Data Matrix codes will optimize existing digital resources in the field of pharmaceutical care for citizens with rare diseases at the regional level.
Keywords
For citations:
Socolova O.V., Isaeva I.Yu., Korzina N.S. Digitalization of pharmaceutical care for children with rare diseases. Patient-Oriented Medicine and Pharmacy. 2025;3(3):107-115. (In Russ.) https://doi.org/10.37489/2949-1924-0109. EDN: TTNEGJ
Background
In the current healthcare landscape, drug provision for children with rare diseases is an integral part of the state-subsidized pharmaceutical benefit system [1]. The increasing number of patients with rare diseases in the regions of the Russian Federation [2] necessitates the digitalization of interaction mechanisms between government bodies, medical, and pharmaceutical organizations. The digitalization and standardization of business processes in the drug supply chain will enhance the transparency, efficiency, and personalization of drug provision. This is expected to overcome practical challenges and mitigate risks in the implementation of treatment for children with rare diseases at the regional level.
Objective
To examine the features of digitalizing drug provision for children with rare diseases at the regional level.
Tasks
To analyze the state of drug assistance for children with rare diseases in the Yaroslavl region.
To identify cases of rare diseases diagnosed and registered within the region.
To study the digital business processes in the supply chain of medicinal products used for treating rare diseases.
Methodology
The study was conducted in several stages. The initial stage involved an analysis of regional drug provision for children with rare diseases. The second stage included the identification of rare disease cases registered in the Yaroslavl region and the medicinal products for their treatment. The subsequent stage examined the current state of digitalization of business processes for the supply of medicinal products (MPs) delivered to the region under the auspices of the "Krug Dobra" (Circle of Kindness) Foundation.
A set of scientific methods was applied, including systemic, logical, and structural analysis. The objects of the study were regulatory legal acts, the International Classification of Diseases (ICD-10), the State Register of Medicinal Products (GRLS), the Unified Register of Registered Medicinal Products of the Eurasian Economic Union, regional and institutional commodity-accounting systems (TAS), and analytical data from the official websites of authorities regulating rare diseases and the Foundation for Supporting Children with Severe Life-Threatening and Chronic Diseases, including Rare Diseases (the "Krug Dobra" Foundation).
Results and Discussion
Drug provision for children with severe life-threatening and chronic diseases, including rare diseases, is facilitated through the "Krug Dobra" Foundation [1]. The Ministry of Health of the Yaroslavl Region effectively collaborates with the Foundation. An analysis of the Foundation's reports from 2021-2024 [2] revealed a growing trend in the provision of drug assistance to children with rare diseases in the region (Fig. 1).
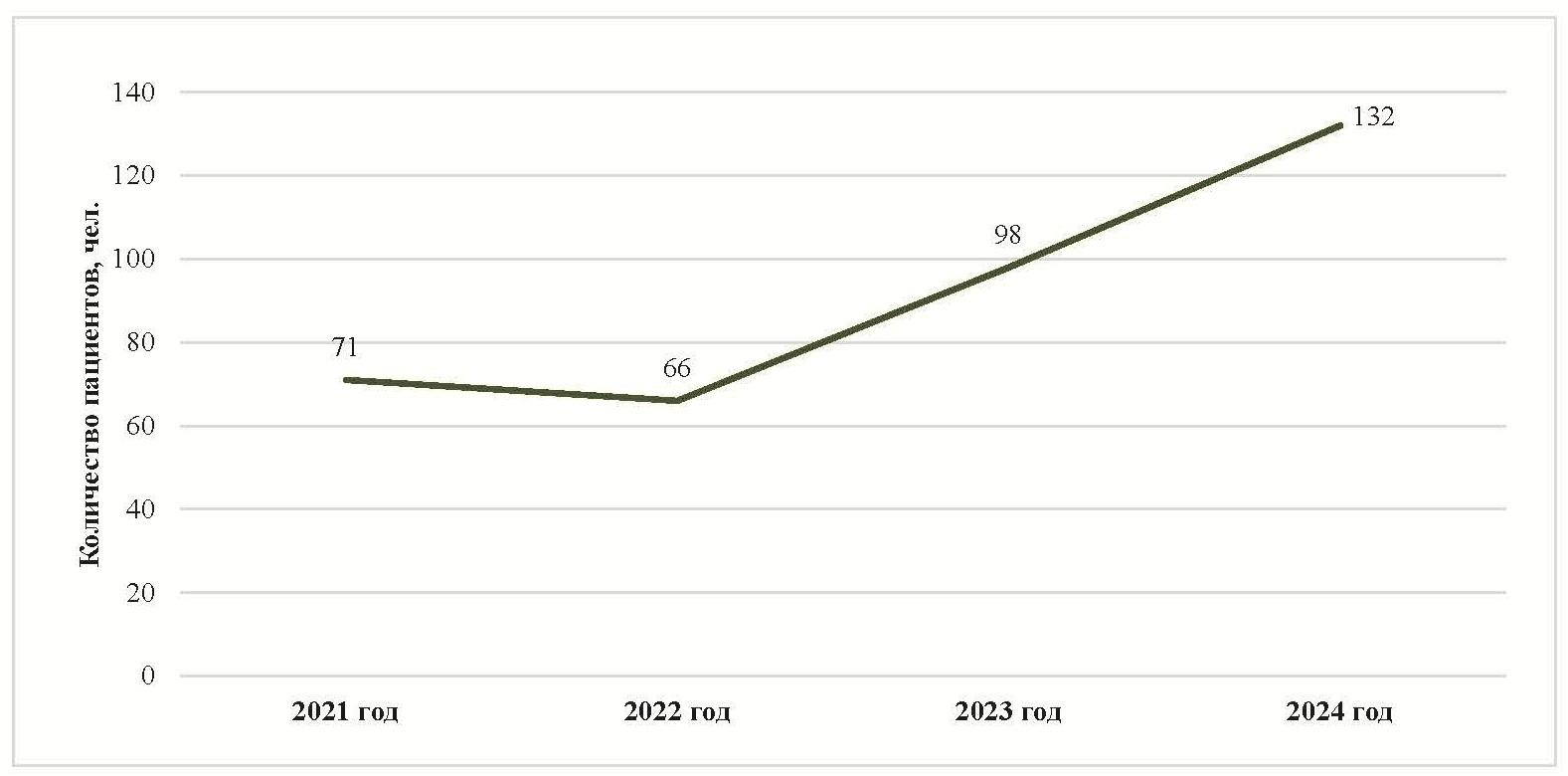
Fig. 1. Statistical data on patients with rare diseases provided with drug therapy
As seen in Fig. 1, over the 4 years of the Foundation's activity, the number of patients requiring treatment for rare diseases in the Yaroslavl region has nearly doubled. This indicates increased awareness among primary healthcare providers in the region [3], resulting in earlier diagnosis and the provision of drug therapy to a larger number of children, which aligns with the goals of both the Foundation and regional healthcare.
As of the beginning of 2024, 228 patients with rare diseases were registered in the Yaroslavl region, 138 of whom were children. A total of 150 individuals required drug therapy. In 2024, over 149 million rubles were allocated from the regional and federal budgets for the drug supply for these patients, with almost half of this amount intended for the procurement of MPs for children [4].
Regulatory documents govern the interaction of healthcare authorities, medical and pharmaceutical organizations of various levels and specializations, as well as individual healthcare professionals in providing medical care to children with rare diseases [5, 6]. It was shown that these patients receive drug therapy based in medical organizations (MOs), and their treatment tactics are coordinated with the chief external specialists of the regional Ministry of Health and specialists from federal MOs. Decisions on MP prescription are made on a personalized basis, allowing for a rapid response to patient needs and minimizing treatment risks.
It should be noted that, in accordance with a presidential program, drug assistance funded by the Foundation is also provided to patients under the age of 19 [7]. Table 1 presents data on the number of patients whose support from the Foundation is ending [8].
Table 1. Number of patients of the "Krug Dobra" Foundation in the Yaroslavl region who have reached the age of 19, by year
| Year | Number of Patients, persons | Estimated Funding Volume, million RUB |
|---|---|---|
| 2024 | 7 | 1,300.00 |
| 2025 | 6 | |
| 2026 | 8 | |
| 2027 | 5 |
According to Table 1, the regional healthcare executive authorities keep records of patients who have aged out of the Foundation's support (reached the age of 19). This ensures continuity of drug therapy as a patient with a rare disease transitions from the pediatric to the adult age group [9], with funding for drug assistance for this category of citizens provided by the regional budget of the Yaroslavl region. Thus, at the regional level, drug provision is ensured for patients with rare diseases, both children and those who have reached adulthood, aimed at the timeliness and effectiveness of treatment.
Next, cases of rare diseases diagnosed and registered in children and patients who have reached the age of 18 in the Yaroslavl region, as well as the drug therapy used to treat these diseases, were studied.
Based on reports from public sources, it was established that 15 out of the 17 nosologies included in the "List of Life-Threatening and Chronic Progressive Rare Diseases Leading to a Reduction in Life Expectancy or Disability" [10] have been identified in the Yaroslavl region. Medical care for patients with rare diseases in the region is provided in accordance with regulations on the organization of medical care by its types, procedures for providing medical care, considering medical care standards, and based on clinical recommendations [11]. However, clinical recommendations are lacking for some rare diseases [3, 6]. Table 2 presents a number of rare diseases according to the ICD-10 classification [12], registered in the Yaroslavl region, for which children and patients over 18 years of age receive appropriate treatment under the "Krug Dobra" Foundation.
Table 2. Rare diseases registered in the Yaroslavl region
| Life-Threatening and Chronic Progressive Rare Disease... | ICD-10 Code | Medicinal Product (INN) |
|---|---|---|
| Spinal Muscular Atrophy (SMA) | G12.1 | Nusinersen |
| X-linked hypophosphatemia | E83.3 | Burosumab |
| Primary immunodeficiency | D81.1 | Immunoglobulin (human) |
| Shereshevsky-Turner syndrome | Q96.8 | Somatropin |
Table 2 also presents the International Nonproprietary Names (INN) of the MPs used to treat these diseases. Subsequently, a search for the state registration of these MPs was conducted on the official sites of registered medicinal products in Russia and the Eurasian Economic Union (EAEU) [13, 14]. In accordance with Part 2, Article 47 of Federal Law No. 61-FZ dated April 12, 2010, "On the Circulation of Medicinal Products," MPs imported into the Russian Federation must be included in the State Register of Medicinal Products or the Unified Register of Registered Medicinal Products of the EAEU. The results of the analysis are presented in Table 3.
Table 3. Information on state registration of medicinal products used to treat rare diseases
| Trade Name (INN) | Dosage Form | Manufacturer | State Registration | |
|---|---|---|---|---|
| RF | EAEU | |||
| SpINRAZA (Nusinersen) | solution for intrathecal injection, 2.4 mg/ml, 5 ml | Netherlands, Biogen Netherlands B.V. | Yes | Yes |
| Lantensens® (Nusinersen) | Russia, JSC "Generium" | |||
| Crysvita® (Burosumab) | solution for injection 20 mg/ml, 1 ml | USA | No | No |
| Cutaquig® (Immunoglobulin human)) | solution for injection 165 mg/ml, 48 ml | Austria, Octapharma | No | No |
| Rastan® (Somatropin) | solution for subcutaneous injection 5 mg/ml, 3 ml | JSC "Pharmstandard-UfaVITA" | Yes | Yes |
As can be seen from Table 3, not all MPs are registered in the Russian Federation and the EAEU. According to Part 5, Article 13, and Parts 3, 3.2 of Article 47 of Federal Law No. 61-FZ "On the Circulation of Medicinal Products," MPs imported into the Russian Federation for providing medical care on vital indications for a specific patient, based on a permit issued by the authorized federal executive body, are not subject to state registration. Furthermore, this law defines the procedure for importing unregistered MPs. Consequently, data on these unregistered MPs are not entered into the Federal State Information System for Monitoring the Movement of Medicinal Products (FGIS MDLP).
The next stage of the research involved an analysis of the digitalization of business processes for the supply chain of MPs delivered to the Yaroslavl region under the "Krug Dobra" Foundation. Federal legislation and regulatory acts of the constituent entities of the Russian Federation influence the functioning of these processes in each region [15, 16, 17, 18].
It was established that the business processes for interaction among participants in drug provision for children with rare diseases in the Yaroslavl region operate at the federal, regional, and institutional levels. However, an analysis of regional and institutional TAS revealed that not all business processes function in a digital format (Fig. 2).
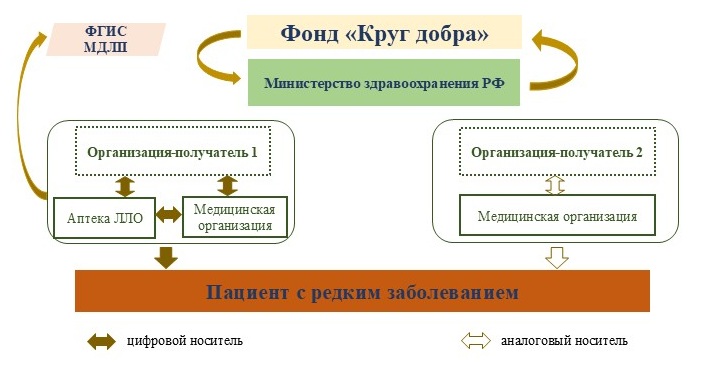
Fig. 2. Diagram of digital interaction between participants in drug provision for children with rare diseases
Data from Fig. 2 indicate that at the current stage, in the Yaroslavl region, medicinal products with state registration and entered into the FGIS MDLP are supplied to "Recipient Organization 1." These MPs are then delivered to pharmacy organizations (POs), which dispense them to privileged categories of citizens or to MOs based on a supply decision and the needs of attached patients [19].
In authorized POs, the dispensing of these MPs is carried out using an electronic prescription, with all digital operations reflected in the regional TAS "1C:LLO" and the FGIS MDLP.
It is known that MOs provide medical care in both outpatient and inpatient settings. In the former case, patients receive MPs based on delivery notes and transfer acts. The transfer of MPs is carried out by the MO where the child with a rare disease is receiving outpatient treatment to the child's legal representative or to the child who has reached the age of 18. In the latter case, if the MP is administered in an inpatient setting, the head nurse of the structural unit formalizes the receipt of the MP via a delivery note and records the supply chain operations in the local TAS "1C:Hospital Pharmacy" using electronic document management (EDM) and in the MO's personal account in the FGIS MDLP.
Consequently, the business processes for the supply chain of registered MPs used to treat children with rare diseases under the "Krug Dobra" Foundation are recorded in a digital format.
Next, we analyzed the supply chain of MPs not registered in the Russian Federation, intended for patients of the Foundation. It was established that in the Yaroslavl region, such MPs are delivered to "Recipient Organization 2."
It should be noted that the issue of expanding the possibilities for handling unregistered MPs [20] in cases of patient recovery or death remains acute.
Since there is no standardized approach to creating a list of unregistered MPs, data entry into the MO's TAS is performed based on a "Product Card" created manually by a pharmaceutical or medical professional. This increases the risks associated with translating the MP name from a foreign language and generating summary reports on analog media.
Therefore, the exchange of information on the MP supply chain among participants in drug provision for children with rare diseases is conducted digitally for registered MPs. This allows for a personalized approach with the recording of all business processes: prescription, issuance of an electronic prescription or request, segregated accounting, dispensing, and withdrawal from civil circulation. In contrast, the supply chain of unregistered MPs is recorded manually by the participants, which creates a likelihood of errors in MP procurement, an increase in routine operations, and, consequently, higher risks in treatment delivery.
Conclusion
The results of the study identified the features of digitalizing regional drug provision for children with rare diseases, using the Yaroslavl region as an example. The regional Ministry of Health, in collaboration with the "Krug Dobra" Foundation, provides drug assistance to patients with orphan diseases across 15 nosologies. Treatment is conducted using MPs that are both registered and unregistered in the Russian Federation, with the import of the latter carried out in accordance with Russian legislation. Consequently, in the Yaroslavl region, supplies of MPs for treating this category of citizens are handled by two authorized recipient organizations. Regional healthcare provides support for one year after these children reach the age of 18, provided they received such support from the Foundation before reaching that age.
The identified features indicate that a specific mechanism for digitalizing business processes for working with patients with rare diseases has been developed, and regions play a significant role in supporting their drug provision. Improving the regulatory framework for digital transformation and standardizing the list of MPs lacking state registration, based on digital information from barcodes or Data Matrix codes, will optimize existing digital resources in the field of drug provision for citizens suffering from rare diseases at the regional level.
References
1. Decree of the President of the Russian Federation No. 16 dated January 5, 2021 "On the Establishment of the Circle of Kindness Fund for Supporting Children with Severe Life-Threatening and Chronic Diseases, Including Rare (Orphan) Diseases" (Electronic resource). (accessed: 06/28/2025) https://www.consultant.ru/document/cons_doc_LAW_373493/.
2. Annual Reports of the Circle of Kindness Foundation. (Electronic resource). (In Russ.) (accessed: 06/28/2025) https://xn--80abfdb8athfre5ah.xn--p1ai/%D0%BE-%D1%84%D0%BE%D0%BD%D0%B4%D0%B5/%D0%B3%D0%BE%D0%B4%D0%BE%D0%B2%D1%8B%D0%B5-%D0%BE%D1%82%D1%87%D0%B5%D1%82%D1%8B-%D1%84%D0%BE%D0%BD%D0%B4%D0%B0/
3. Annual Bulletin of the Expert Council on Rare (Orphan) Diseases. V. 2 – M., 2024. – 324 p. (Electronic resource). (In Russ.) (accessed: 06/28/2025) blok_white-book2024-2b.indd
4. February 28 is the Day of Rare Diseases. (Electronic resource). (In Russ.) (accessed: 06/28/2025) https://portal.yarregion.ru/depts-zdrav/press-center/news/detail.php?SECTION_CODE=&ELEMENT_CODE=vse-zhiteli-regiona-stradayushchie-redkimi-zhizneugrozhayushchimi-zabolevaniyami-poluchayut-neobhodimuyu-medicinskuyu-pomoshch--&sphrase_id=9431.
5. Annual Bulletin of the Expert Council on Rare (Orphan) Diseases. State Duma Committee on Health. Moscow, 2022. (Electronic resource). (In Russ.) (accessed: 06/28/2025) maket_white-book2022-23_4.indd.
6. Annual Bulletin of the Expert Council on Rare (Orphan) Diseases. V.1 – M., 2024. – 304 p. (Electronic resource). (In Russ.) (accessed: 06/28/2025) blok_white-book2024-1.indd
7. Komarov I. A., Gevorkyan A. K. Issues of organizing drug provision for children who received treatment through the Circle of Kindness Foundation upon reaching the age of majority. Bulletin of the N.A. Semashko National Research Institute of Public Health. 2024;28(4):110-115. (In Russ.) DOI: 10.69541/NRIPH.2024.04.017. – EDN: PMTQSV.
8. Yaroslavl residents with rare diseases need half a billion rubles for medicine. (Electronic resource). (In Russ.) (accessed: 06/28/2025) https://www.yarnews.net/news/show/yaroslavl-region/73498/na_lekarstva_yaroslavcev_s_redkimi_boleznyami_nuzhno_polmilliarda_rublej.htm.
9. Kavkaeva K. P., Malysheva A. A. The Circle of Kindness Foundation: Prerequisites for Creation and the Specificity of Financial and Legal Regulation. Russian Law Journal. 2024;(1):167-176. (In Russ.) DOI: 10.34076/20713797_2024_1_167. – EDN: JMBEDB.
10. Government Decree No. 403 of April 26, 2012 "On the Procedure for Maintaining the Federal Register of Persons Suffering from Life-Threatening and Chronic Progressive Rare (Orphan) Diseases that Reduce the Life Expectancy of Citizens or Cause Their Disability, and Its Regional Segment" (together with the "Rules for Maintaining the Federal Register of Persons Suffering from Life-Threatening and Chronic Progressive Rare (Orphan) Diseases that Reduce the Life Expectancy of Citizens or Cause Their Disability, and Its Regional Segment"). (Electronic resource). (In Russ.) (accessed: 06/28/2025) https://base.garant.ru/70168888/?ysclid=mcubz0p-sus915701031.
11. Federal Law No. 323-FZ of November 21, 2011 "On the Fundamentals of Protecting the Health of Citizens in the Russian Federation". (Electronic resource). (In Russ.) (accessed: 06/28/2025) https://www.consultant.ru/document/cons_doc_LAW_121895/.
12. International Statistical Classification of Diseases and Health Problems (10<sup>th</sup> Revision) (ICD-10) (version 2.27 dated 02. 09. 2024). (Electronic resource). (In Russ.) (accessed: 07/08/2025) https://www.consultant.ru.
13. Gosudarstvennyj Reestr lekarstvennyh sredstv. Elektronnaya versiya. State Register of Medicines. (Electronic resource). (In Russ.) (accessed: 06/28/2025) URL: http://grls.rosminzdrav.ru.
14. Unified Register of Registered Medicines of the Eurasian Economic Union. (Electronic resource). (In Russ.) (accessed: 06/28/2025) https://portal.eaeunion.org/sites/commonprocesses/ru-ru/Pages/DrugRegistrationDetails.aspx/.
15. Decree of the Government of the Russian Federation No. 769 dated May 21, 2021 "On Approval of the Rules for Providing Medical Care (if necessary, outside the Russian Federation) to a specific child with a severe life-threatening or chronic disease, including a rare (orphan) disease, or to groups of such children". (Electronic resource). (In Russ.) (accessed: 06/28/2025) https://www.consultant.ru/document/cons_doc_LAW_384635/.
16. Decree of the Government of the Russian Federation No. 555 dated April 8, 2021 "On Approval of the Rules for Maintaining an Information Resource Containing Information about Children with Severe Life-Threatening and Chronic Diseases, Including Rare (Orphan) Diseases, Including Information about the Purchase of Medicines and Medical Devices for Such Children, Including Those Not Registered in the Russian Federation, and Technical Rehabilitation Devices, and Information about the Results of Treatment for Such Children". (Electronic resource). (In Russ.) (accessed: 06/28/2025) https://www.consultant.ru/document/cons_doc_LAW_381889/5de780a21dc683f26e3012193172c-3b1c4979af6/.
17. Order No. 970n of the Ministry of Health of the Russian Federation dated October 6, 2021, "On Approval of the Procedure for Monitoring the Movement and Accounting of Medicines and Medical Devices Purchased for a Specific Child with a Severe Life-Threatening or Chronic Disease, Including a Rare (Orphan) Disease, or for Groups of Such Children, and (or) Their Redistribution among Recipient Organizations. ". (Electronic resource). (In Russ.) (accessed: 06/28/2025) https://www.consultant.ru/document/cons_doc_LAW_400206/2ff7a8c72de3994f30496a0ccbb1ddafdaddf518/.
18. Samoshchenkova I.F., Garankina R. Yu., Snimshchikova I.A. et al. "The Circle of Kindness" as part of the drug provision for patients with orphan diseases. Vestnik Smolenskoy gosudarstvennoy meditsinskoy akademii. 2022.;21(2):220-229. (In Russ.) DOI: 10.37903/vsgma.2022.2.28. – EDN: TUIUXE.
19. Government Decree No. 545 dated April 6, 2021, "On the Procedure for Acquiring Medicines, Medical Devices, and Technical Rehabilitation Equipment for a Specific Child with a Severe Life-Threatening or Chronic Disease, Including a Rare (Orphan) Disease, or for Groups of Such Children". (Electronic resource). (In Russ.) (accessed: 06/28/2025) https://www.consultant.ru/document/cons_doc_LAW_381890/.
20. Semenova A.A., Gaisarov A.Kh., Kononova I.V., Ibragimova G.Ia. Analysis of the legal regulation of the determination of the dispensing conditions of unregistered medicines in the Russian Federation. Medical & pharmaceutical journal “Pulse”. 2023;25(1):72-77. (In Russ.) doi: 10.26787/nydha-2686-6838-2023-25-1-72-77.
About the Authors
O. V. SocolovaРоссия
Olga V. Socolova, Cand. Sci. (Pharm.), Associate Professor, Associate Professor at Department
Department of Management and Economics of Pharmacy
Yaroslavl
Competing Interests:
Authors declare no conflict of interest requiring disclosure in this article
I. Yu. Isaeva
Россия
Ilona Yu. Isaeva, head of pharmacy
Yaroslavl
Competing Interests:
Authors declare no conflict of interest requiring disclosure in this article
N. S. Korzina
Россия
Nadezhda S. Korzina, Deputy Minister — Head of the Department
Department of Digital Transformation and Project Activities
Yaroslavl
Competing Interests:
Authors declare no conflict of interest requiring disclosure in this article
Review
For citations:
Socolova O.V., Isaeva I.Yu., Korzina N.S. Digitalization of pharmaceutical care for children with rare diseases. Patient-Oriented Medicine and Pharmacy. 2025;3(3):107-115. (In Russ.) https://doi.org/10.37489/2949-1924-0109. EDN: TTNEGJ
JATS XML


































.png)
